Deciphering its Role in Congenital Adrenal Hyperplasia
Congenital adrenal hyperplasia (CAH) is a group of inherited disorders characterized by the impaired production of cortisol and, in some cases, aldosterone by the adrenal glands. This hormonal imbalance triggers a cascade of physiological disruptions, often presenting with ambiguous genitalia in newborns, excessive salt loss, and developmental delays. At the heart of this enigmatic condition lies ACTH, a hormone with a complex and paradoxical role in CAH.
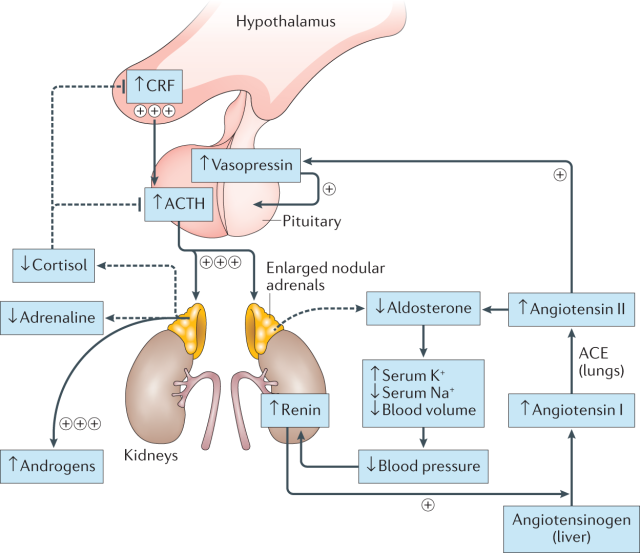
The Master Regulator: ACTH in Healthy Individuals
ACTH, or adrenocorticotropic hormone, is secreted by the pituitary gland in response to corticotropin-releasing hormone (CRH) from the hypothalamus. This hormonal cascade acts as a stress response system, prompting the adrenal glands to produce cortisol, a crucial hormone for regulating metabolism, blood pressure, and immune function. In healthy individuals, a negative feedback loop exists, where rising cortisol levels suppress ACTH production, maintaining hormonal homeostasis.
The Unbalanced Equation: ACTH in CAH
In CAH, genetic mutations disrupt the normal functioning of enzymes involved in cortisol and aldosterone biosynthesis. This enzymatic deficiency leads to insufficient hormone production, triggering the hypothalamus-pituitary-adrenal axis into overdrive. The pituitary gland, sensing low cortisol levels, ramps up ACTH secretion in an attempt to stimulate the adrenal glands. However, due to the enzymatic defect, the adrenal glands remain sluggish in their hormone production, creating a paradoxical situation of high ACTH and low cortisol/aldosterone levels.
The Enigma Unfolds: ACTH’s Diverse Effects in CAH
ACTH’s role in CAH extends beyond its futile attempt to stimulate cortisol production. It exerts diverse effects on various tissues, contributing to the multifaceted clinical picture of the condition.
- Stimulation of adrenal androgen production: In the absence of proper cortisol synthesis, ACTH shunts its focus towards stimulating the production of adrenal androgens, such as testosterone. This androgen excess can lead to virilization in females, including clitoral enlargement and labioscrotal fusion.
- Suppression of sex hormone production: High ACTH levels can suppress the production of sex hormones from the gonads, contributing to delayed puberty and impaired fertility in both sexes.
- Bone mineral density issues: Chronically elevated ACTH levels can interfere with bone metabolism, leading to osteoporosis and increased fracture risk.
- Metabolic and growth disturbances: ACTH can directly influence carbohydrate and fat metabolism, contributing to weight gain and insulin resistance. Additionally, it can hinder linear growth and development in children.
Deciphering the Enigma: Implications for CAH Management
Understanding the complex interplay between ACTH and its diverse effects in CAH is crucial for effective management. Treatment regimens typically involve:
- Glucocorticoid replacement: Administering synthetic cortisol to mimic its physiological functions and suppress ACTH production. This helps normalize metabolism, electrolyte balance, and prevent virilization.
- Mineralocorticoid replacement (if needed): In some CAH forms, aldosterone deficiency also occurs, necessitating replacement therapy to maintain electrolyte balance and prevent salt loss.
- Surgery: In cases of severe genital ambiguity, corrective surgery may be recommended after thorough counseling and informed consent.
The Evolving Landscape: Research and Future Directions
Research on ACTH’s role in CAH is ongoing, with a focus on:
- Understanding the mechanisms by which ACTH exerts its diverse effects beyond cortisol stimulation.
- Developing novel therapeutic strategies to target specific ACTH-mediated pathways and potentially reduce glucocorticoid dependence.
- Personalized medicine approaches to tailor treatment based on individual variations in ACTH sensitivity and metabolism.
Unpacking the CAH Mosaic: A Closer Look at Different Forms
CAH is a constellation of disorders categorized by specific enzyme deficiencies. Here’s a glimpse into some key forms:
- 21-Hydroxylase Deficiency (21-OHD): The most common form, impacting cortisol and aldosterone production. High ACTH levels stimulate androgen production, causing virilization in females and precocious puberty in males.
- 11-beta-Hydroxylase Deficiency: Characterized by cortisol deficiency but normal aldosterone production. Symptoms may be milder than 21-OHD, with salt wasting appearing only in severe cases.
- 3beta-Hydroxysteroid Dehydrogenase Deficiency: This rare form disrupts cortisol and all steroid hormone production, leading to severe salt wasting and ambiguous genitalia in both sexes.
Beyond Cortisol: Unveiling ACTH’s Multifaceted Impact
The impact of high ACTH goes beyond simply attempting to stimulate cortisol production. Here’s a deeper dive into its diverse effects:
- Interfering with sex hormone production: Chronically elevated ACTH suppresses the release of gonadotropins, crucial hormones for sex hormone production. This can lead to delayed puberty, menstrual irregularities, and even infertility in both sexes.
- Skeletal woes: Chronic ACTH exposure can hinder bone mineralization, leading to osteoporosis and increased fracture risk, particularly in adults with poorly controlled CAH.
- Metabolic mayhem: ACTH can directly influence carbohydrate and fat metabolism, contributing to weight gain, insulin resistance, and even diabetes in some cases. This metabolic dysregulation can further complicate CAH management.
- Neurological whispers: Emerging research suggests a link between ACTH and cognitive function in CAH. Some studies have shown associations between high ACTH levels and learning difficulties, highlighting the need for further investigation into potential neurocognitive effects.
Emerging Strategies: Tackling the ACTH Enigma with Innovation
The quest to navigate the ACTH enigma continues, with researchers exploring novel therapeutic approaches:
- Targeted therapies: Investigating specific ACTH-mediated pathways that contribute to certain CAH symptoms, like bone issues or metabolic disturbances. This could pave the way for therapies that address these concerns beyond simply managing cortisol levels.
- Personalized medicine: Utilizing genetic and metabolic profiling to tailor treatment regimens based on individual differences in ACTH sensitivity and response. This personalized approach holds promise for optimizing therapy and minimizing side effects.
- Gene therapy: While still in its early stages, gene therapy approaches aim to correct the underlying genetic defects in CAH, potentially offering a cure for this lifelong condition.
Living Beyond the Enigma: Empowering Individuals with CAH
The journey with CAH presents its unique challenges, but understanding the complexities of the ACTH enigma empowers individuals and their healthcare providers to make informed decisions about treatment and management. With ongoing research and evolving therapeutic strategies, the future for individuals with CAH holds the promise of improved quality of life and long-term health outcomes.
The ACTH enigma in CAH highlights the intricate interplay between hormones and their multifaceted effects on the human body. Deciphering this enigma is essential for developing effective management strategies and improving the quality of life for individuals living with CAH. As research continues to unravel the complexities of this condition, the hope lies in personalized approaches that target the specific needs of each patient, ultimately empowering individuals to thrive beyond the challenges of CAH.
